A team aiming to accelerate the development of their MD product or,
A production unit that wants to anticipate problems,
Company already established,
Currently involved in the development of a MD product (safe IVD),
Above TRL3 / CRL3,
Open to WSL members and non-members.

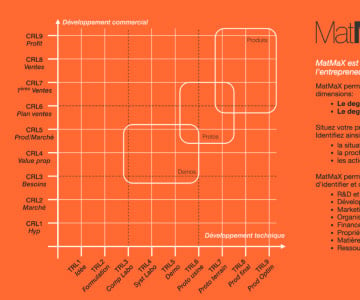
You will reach the CE marking with less trouble, which will allow you to jump from a Commercial Readiness Level 4 (CRL4) to a Commercial Readiness Level 6 (CRL 6) with more confidence.
ISO certification and CE marking are not only the "holy grail" for product commercialization. They increase the value of your company and establish good practices to produce and market high-safety medical product.
Actually, a medical device needs a long development process (TRL2 to TRL8) before arriving on the market, which in turn implies the CE marking (TRL8). That is the only way to access customers (CRL6) and collect the first revenues.

Ten Workshops (WS) which address the “Technical Readiness Level” topics (TRL) enabling your product to reach CE marking under the best possible conditions.
Following all WS (or only some of them: “à la carte” program) you will offer your target client the best product earlier.
Several experts in TRL topics will support you in each step you take. The general framework will be addressed during the first half-day, in which pivotal issues will be covered.
The second half-day will be focused on your own product matters development. TRL-B ME offers an exclusive access to expert knowledge and valuable resources which enable to make the process smooth and hassle-free.
The last WS (WS11) opens up business and partnership opportunities with some of the main active countries in MedTech.

TRL Booster MedTech Edition 2024 (TRL-B ME) is neither an accelerator program to prepare your company to pitch for funding or a contest, nor a training to improve your business model.
Field experts will help you identify, determine and solve issues related to the development of your own product.
TRL-B ME will not discuss neither general product development, nor all-purpose model but your own development risks within our company.
TRL-B ME will delve deeply into the agenda topics to understand the stumbling blocks, challenges and critical aspects of product development.
TRL-B ME will enable you to meet Belgian and European Notified Bodies, as well as AFMPS representatives, in a manner that will help you prepare to effectively interact with authorities (WS9).