Optimize the development of medical devices and rapidly reach CE marking
The TRL Booster MedTech Edition offers ten workshops to optimize the development of medical devices and rapidly obtain CE marking, by minimizing obstacles and facilitating market deployment.

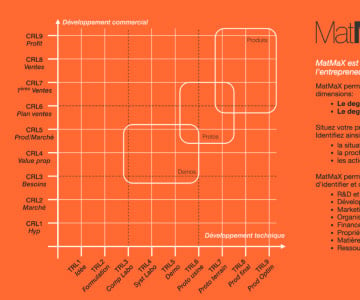
The ten main phases of a medical device life cycle
The medical device design and development process is a complex and interconnected series of steps. It is a long way down the road to CE marking. Each step has to be planned and executed carefully, in collaboration with various stakeholders. Going through the different stages of the regulatory process for medical devices and product development is always filled with pitfalls which impair the product market release.
In this TRL Booster MedTech Edition (TRL-B ME), we examine the first ten phases of a medical device lifecycle which are essential in getting your product to the market quickly and efficiently.
TRL-B ME aims to give you the best chances of implementing your product in less delay, through diligent work, with well-prepared documentation and an adequate structure for market launch.

Ten Workshops (WS) which address the “Technical Readiness Level” topics (TRL) enabling your product to reach CE marking under the best possible conditions. Following all WS (or only some of them: “A la carte” program) you will offer your target client the best product earlier. Several experts in TRL topics will accompany in each step you take. The general framework will be addressed during the first half a day, in which pivotal issues will be covered. The second half a day will be focused on your own product matters development. TRL-B ME offer an exclusive access to expert knowledge and valuable resources which enable to make the process smooth and hassle free. The last WS (WS11) opens up business and partnership opportunities with some of the main active countries in MedTech.

TRL Booster MedTech Edition 2024 (TRL-B ME) is neither an accelerator program to prepare your company to pitch for money or contest, nor a training to improve your business model. Field experts will help you to identify, determine and solve issues in your own development product. TRL-B ME will not discuss neither general product development, nor all-purpose model but your own development risks within our company. TRL-B ME will go deeply in the agenda topics (see under) to understand stumbling blocks, challenges and critical aspects of MD process. TRL-B ME will enable you to meet Belgian and European Notified Bodies, in such a way that you can prepare yourself to take with authorities in an efficient way (WS9). TRL-B ME will give an introduction to foreseen you company at international level as far as certification barriers are concerned (WS10). TRL-B ME will introduce you in MedTech ecosystem in neighbour countries (WS11)

A team aiming to accelerate their MD product development or, A production unit that wants to anticipate problems, Already established company, Currently involved in MD product development (safe IVD), Above TRL3 / CRL3, Open to IGNITY’ s members and non-member.

You will reach the CE marking with less trouble in order to jump from a Commercial Readiness Level 4 (CRL4) to a CRL 6 with more confidence. ISO certification and CE marking are not only the “holy grail” for product commercialization. They increase the value of your company and establish good practices to produce and release high level of safety medical product. Actually, a medical device needs a long development process (TRL2 up to TRL8) before arriving in the market, which in turn implies the CE marking (TRL8). That is the only way to access customers (CRL6) and collect the first revenues.

10 days of exchanges and intensive collaboration
10 workshops spread over 8 months
Or any professional in the sector
How much does it cost?
for the first participant - 220€/WS for the second participant of the same company.
for the first participant - 260€/WS for the second participant of the same company.
NB1: The price includes the catering and training materials.
NB2: Registration is confirmed after payment of the invoice. Registration fees are non-refundable in the event of the company representative(s)' absence.
The coaches are all engineers with proven experience in the creation and launch of tech startups.
With the support of